This page is part of the ICVP (v0.2.0: Releases Draft) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 0.3.0. For a full list of available versions, see the Directory of published versions
Example QuestionnaireResponse: ICVPQRExample
Profile: SDC Questionnaire Response
Tag: lformsVersion: 33.3.6 (Details: [not stated] code lformsVersion: 33.3.6)
| LinkID | Text | Definition | Answer |
|---|---|---|---|
 | Questionnaire:ICVP Model Questionnaire | ||
 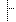 | Full Name of the client | John Doe | |
 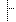 | Date of Birth | 2017-05-17 | |
 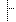 | Sex | administrativeSex M: Male | |
 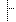 | Nationality | ISO 3166-1 Codes for the representation of names of countries and their subdivisions — Part 1: Country code ATG: Antigua and Barbuda | |
  | National Identification Document | ASVPR24682C | |
  | Parent or Guardian Details | ||
   | Name of Parent or Guardian | Father Doe | |
   | Relationship Status | DVCRelationshipStatus Parent: Parent | |
  | Vaccine Certificate Details | ||
   | Vaccine or Prophylaxis ID | ICVPProductIds YellowFeverProductd8a09f80301dc05e124f99ffe7711fc0: 01/01/1987Yellow FeverSTAMARILVial + Ampoule10Sanofi PasteurAgence nationale de sécurité du médicament et des produits de santé | |
   | Date of Vaccination | 2025-08-08 | |
   | Name of supervising clinician | Mr. Supervisor | |
   | Relevant authoring reponsible for issuing the certificate, or for overseeing the administration center | National Health Institute | |
   | Batch Number | 23543657LOT | |
   | Certificate Validity Period | ||
    | From | 2025-08-15 | |