WHO SMART Trust
1.0.0 - 1.0.0
WHO SMART Trust
1.0.0 - 1.0.0
This page is part of the Trust (v1.0.0: Release) based on FHIR (HL7® FHIR® Standard) R4. This is the current published version in its permanent home (it will always be available at this URL). For a full list of available versions, see the Directory of published versions
This guide describes the specifications and on-boarding procedures for WHO's Global Digital Health Certification Network (GDHCN). The GDHCN is a mechanism to support verification of health documents and certifications that are exchanged between participants of the GDHCN. These health certifications may include COVID-19 certificates, routine immunization cards, and home-based records consistent with International Patient Summary standards. This mechanism provides means of harmonizing global health protocol standards and establishing a system for recognition of digital certificates for continuity of care at point of entry. The GDHCN is designed to leverage existing investments by jurisdictions that were made under the COVID-19 response and provide the digital health infrastructure needed for resiliency in future epidemic and pandemic responses.
The GDHCN is a digital reflection of the trust WHO already has with Member States. The GDHCN is a digital trust network is based on proven concepts which are used to describe the specifications and mechanisms for establishing trust. Eligible participants of the trust network may apply to join by following an on-boarding process. The GDHCN is operated under the GDHCN Administrative and Operational Framework.
Trust Network
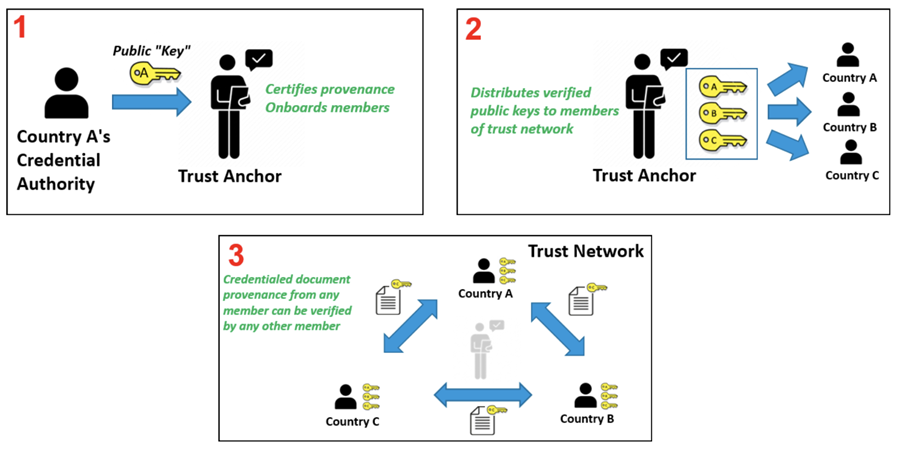
In response to COVID-19, Governments and organizations across the world have developed and adopted standards and technologies to create, present, and verify digital vaccination and test credentials. However, a global technical framework to enable convenient use and interoperability of these credentials between systems – while also allowing domestic autonomy over their use – does not exist yet and is critically needed.
The WHO Global Digital Health Certification Network is a collection of components that are used to verify interoperable digital health documents or certificates. This system of comprised of three main features:
In addition to verifying and validating COVID-19 certificates, a global digital health trust network such as the GDHCN can:
The interoperable exchange of health information in a trusted environment is a complex task with an increasingly large number of stakeholders (e.g. public health agencies, accredited labs, border control organizations, institutions authorized to verify) that need to ensure that data is transferred safely and securely, that the health content is interoperable, and that information is understandable and actionable. This guide details how to utilize a global technical framework to allow interoperability of health credentials between systems, while preserving domestic autonomy over their use.
Achieving global interoperability of health certificates does not require that all jurisdictions use the same standard. Interoperability can also be achieved when there are pre-arranged mechanisms in place so that certificates issued by one jurisdiction are accepted in another. A number of services and technical artifacts have been developed to address particular criteria for establishing interoperability and a system of trust including:
This guide describes expected workflows for potential actors in a trust ecosystem, namely:
The audience for this guide includes decision makers, analysts and technical assets at potential individual issuers, existing trust networks or potential verifiers who may participate in the federated trust network. Stakeholders include Member States, regional networks, and standards development organizations.