WHO SMART Trust
1.1.3 - v1.1.3
WHO SMART Trust
1.1.3 - v1.1.3
This page is part of the Trust (v1.1.3: Release) based on FHIR (HL7® FHIR® Standard) R4. The current version which supersedes this version is 1.4.0. For a full list of available versions, see the Directory of published versions
| Official URL: http://smart.who.int/trust/ImplementationGuide/smart.who.int.trust | Version: 1.1.3 | |||
| Active as of 2024-04-25 | Computable Name: SMART_WHO_INT_TRUST | |||
Contents:
This guide describes the specifications and on-boarding procedures for WHO's Global Digital Health Certification Network (GDHCN). The GDHCN is a mechanism to support verification of health documents and certifications that are exchanged between participants of the GDHCN. These health certifications may include COVID-19 certificates, routine immunization cards, and home-based records consistent with International Patient Summary standards. This mechanism provides means of harmonizing global health protocol standards and establishing a system for recognition of digital certificates for continuity of care at point of entry. The GDHCN is designed to leverage existing investments by jurisdictions that were made under the COVID-19 response and provide the digital health infrastructure needed for resiliency in future epidemic and pandemic responses.
The GDHCN is a digital reflection of the trust WHO already has with Member States. The GDHCN is a digital trust network is based on proven concepts which are used to describe the specifications and mechanisms for establishing trust, which allow eligible participants to establish new trust domains for exchange of verifiable digital health records. Eligible participants of the trust network may apply to join by following an on-boarding process. The GDHCN is operated under the GDHCN Administrative and Operational Framework.
Trust Network
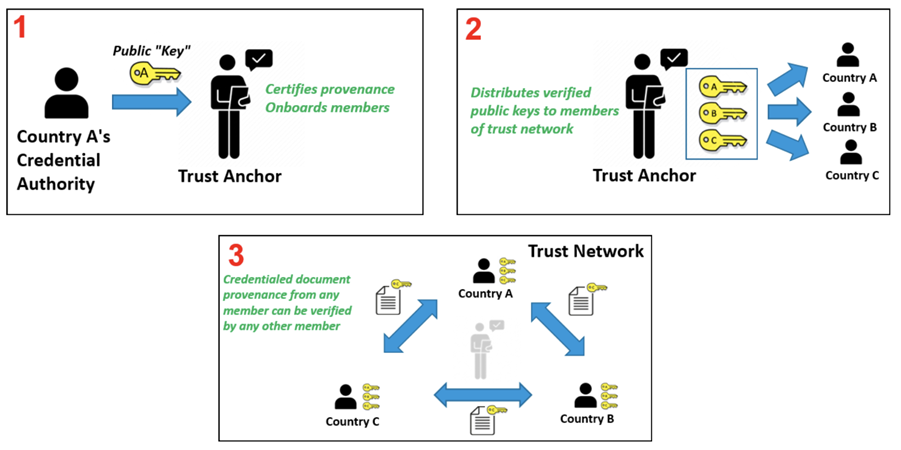
In response to COVID-19, Governments and organizations across the world have developed and adopted standards and technologies to create, present, and verify digital vaccination and test credentials. However, a global technical framework to enable convenient use and interoperability of these credentials between systems – while also allowing domestic autonomy over their use – does not exist yet and is critically needed.
The WHO Global Digital Health Certification Network is a collection of components that are used to verify interoperable digital health documents or certificates. This system of comprised of three main features:
In addition to verifying and validating COVID-19 certificates, a global digital health trust network such as the GDHCN can:
The interoperable exchange of health information in a trusted environment is a complex task with an increasingly large number of stakeholders (e.g. public health agencies, accredited labs, border control organizations, institutions authorized to verify) that need to ensure that data is transferred safely and securely, that the health content is interoperable, and that information is understandable and actionable. This guide details how to utilize a global technical framework to allow interoperability of health credentials between systems, while preserving domestic autonomy over their use.
Achieving global interoperability of health certificates does not require that all jurisdictions use the same standard. Interoperability can also be achieved when there are pre-arranged mechanisms in place so that certificates issued by one jurisdiction are accepted in another. A number of services and technical artifacts have been developed to address particular criteria for establishing interoperability and a system of trust including:
This guide describes expected workflows for potential actors in a trust ecosystem, namely:
The audience for this guide includes decision makers, analysts and technical assets at potential individual issuers, existing trust networks or potential verifiers who may participate in the federated trust network. Stakeholders include Member States, regional networks, and standards development organizations.
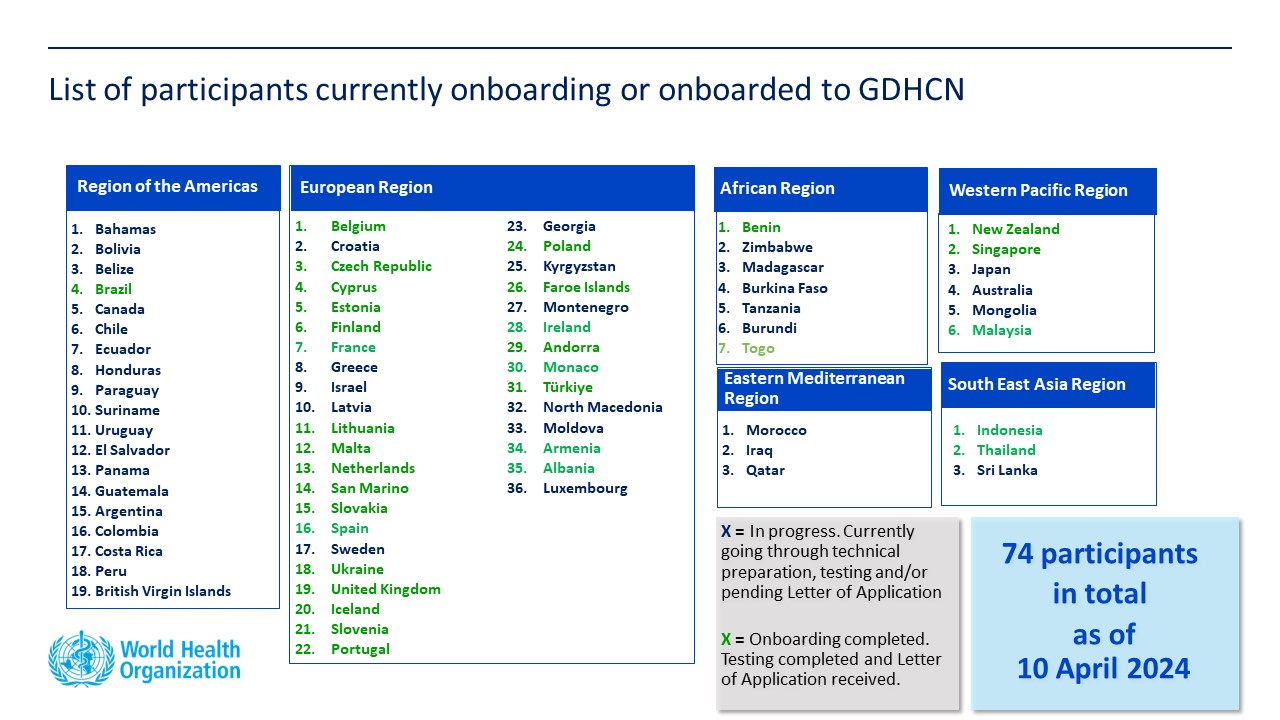
Latest Status
There are no dependencies for this implementation guide. The source code for the WHO SMART Guidelines IG template is available here.
Package hl7.fhir.uv.extensions.r4#1.0.0 This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Sun, Mar 26, 2023 08:46+1100+11:00) |
Package hl7.fhir.uv.ips#1.1.0 International Patient Summary (IPS) FHIR Implementation Guide (built Tue, Nov 22, 2022 03:24+0000+00:00) |
Package hl7.fhir.uv.extensions#5.1.0-cibuild This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Tue, Mar 12, 2024 13:29+1100+11:00) |
Package hl7.fhir.uv.cql#1.0.0-snapshot This implementation guide defines profiles, operations and guidance for the use of CQL with FHIR, both as a mechanism for querying, as well as inline and integrated usage as part of knowledge artifacts. (built Sat, Apr 6, 2024 09:37+1100+11:00) |
Package hl7.fhir.uv.crmi#1.0.0-snapshot This implementation guide defines profiles, operations, capability statements and guidance to facilitate the content management lifecycle for authoring, publishing, distribution, and implementation of FHIR knowledge artifacts such as value sets, profiles, libraries, rules, and measures. The guide is intended to be used by specification and content implementation guide authors as both a dependency for validation of published artifacts, and a guide for construction and publication of content. (built Sat, Apr 6, 2024 06:47+1100+11:00) |
Package hl7.fhir.uv.cpg#current Implementation guidance for creating Clinical Practice Guidelines with formal artifacts to facilitate sharing and implementation of the guideline (built Sun, Apr 14, 2024 19:57+0000+00:00) |
Package who.base#current An empty Implementation Guide to be used as a starting point for building SMART Guidelines Implementation Guides (built Thu, Apr 25, 2024 23:14+0000+00:00) |
Package hl7.fhir.r4.examples#4.0.1 Example resources in the R4 version of the FHIR standard |
Package hl7.fhir.uv.sdc#3.0.0 The SDC specification provides an infrastructure to standardize the capture and expanded use of patient-level data collected within an EHR. |
Package ihe.iti.balp#1.1.1 The Basic Audit Log Patterns (BALP) Implementation Guide is a Content Profile that defines some basic and reusable AuditEvent patterns. This includes basic audit log profiles for FHIR RESTful operations to be used when there is not a more specific audit event defined. A focus is enabling Privacy centric AuditEvent logs that hold well formed indication of the Patient when they are the subject of the activity being recorded in the log. Where a more specific audit event can be defined it should be derived off of these basic patterns. (built Fri, Oct 21, 2022 08:58-0500-05:00) |
Package ihe.formatcode.fhir#1.1.0 Implementation Guide for IHE defined FormatCode vocabulary. (built Thu, Feb 24, 2022 16:55-0600-06:00) |
Package ihe.iti.mhd#4.2.0 ImplementationGuide for IHE IT Infrastructure Technical Framework Supplement Mobile access to Health Documents (MHD) (built Wed, Dec 7, 2022 16:33-0600-06:00) |
Package who.ddcc#current This WHO Digital Documentation of COVID-19 Certificates (DDCC) Implementation Guide details how to use Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR) for consistent digital representation of COVID-19 certificates and interoperability. (built Wed, Apr 24, 2024 00:07+0000+00:00) |
Feedback specific to this Implementation Guide can provided through:
Sign up on chat.fhir.org community and follow the stream who-smart-guidelines for questions, queries and chats related to WHO SMART Guidelines
WHO also hosts weekly calls on authoring and implementing WHO SMART Guidelines where participation is welcome. Please send an email at gdhcn-support@who.int in order to get invited.
The specification herewith documented is a demo working specification, and may not be used for any implementation purposes. This draft is provided without warranty of completeness or consistency, and the official publication supersedes this draft. No liability can be inferred from the use or misuse of this specification, or its consequences.